Inhibition of acrylic acid and acrylate autoxidation
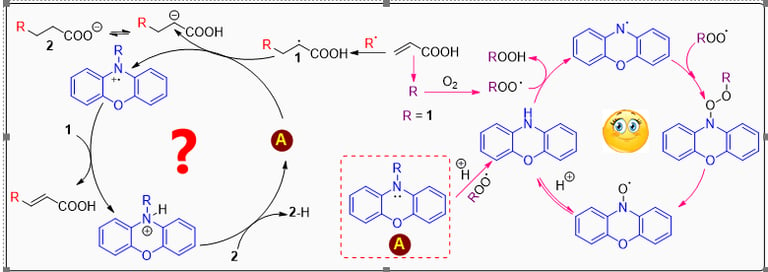
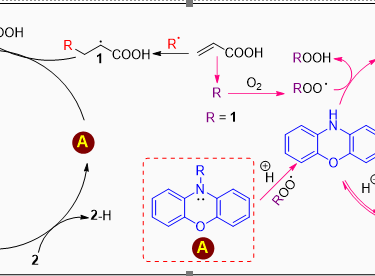
Acrylic acid (AA) is a highly reactive monomer that poses significant challenges for transport and storage due to its tendency for runaway polymerization. To mitigate this risk, inhibitors such as hydroquinone monomethyl ether (MeHQ) and phenothiazine (PTZ) are commonly employed, although these substances exhibit certain limitations. This research utilizes an advanced spectrophotometric approach employing the autoxidizable STY-BODIPY dye to monitor the autoxidation of acrylic acid, n-butyl acrylate, and the non-polymerizable 2-ethylhexanol. The study specifically examines the impact of various radical-trapping antioxidants (RTAs) on these processes. The findings indicate that the radical-trapping stoichiometry is highly substrate-dependent. Notably, N-alkyl derivatives of phenoxazine are identified as highly effective inhibitors of AA autoxidation. This is attributed to their unique mechanism, which minimizes the accumulation of phenoxazine-derived nitroxides, enhancing their radical-trapping capacity. These results strongly suggest that N-alkylated phenoxazine derivatives warrant further investigation as potential stabilizers for acrylic acid.
Org. Biomol. Chem., 2025, Just Accepted
Electrochemically initiated synthesis of ethylene carbonate from CO2
Electrochemical methods have emerged as crucial strategies in the pursuit of achieving net-zero emissions, addressing key objectives such as hydrogen production, CO2 capture and CO2 conversion. Here we propose an electrochemically initiated process that integrates these three tasks while producing valuable industrial chemicals, such as hydrogen, ethylene carbonate and urethane polymers. In this approach, CO2, ethylene and water are transformed into ethylene carbonate and hydrogen, and the key to enabling this transformation is the discovery that CO2, captured as sodium bicarbonate, can be utilized in organic carbonate synthesis in an aqueous solution. Importantly, this transformation is mediated by succinimide, an organic catalyst that interacts with protons, bromine and CO2 throughout this process. Technoeconomic analysis and life-cycle assessment of a commercial-scale process based on our proposed reaction pathway reveals its profitability and environmental viability. In the future, this approach could be generalized for the synthesis of various organic carbonates, with implications for producing lithium battery electrolytes, carbonate polymers and non-isocyanate urethane polymers.
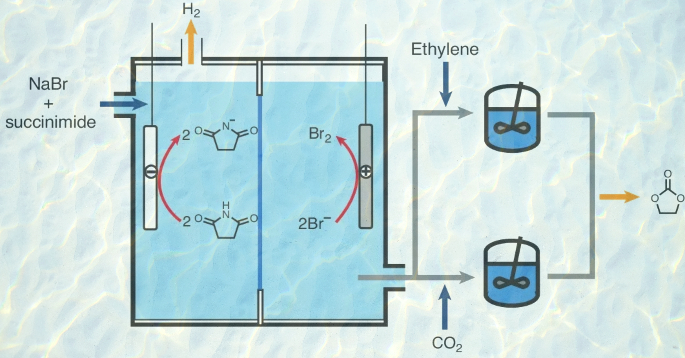
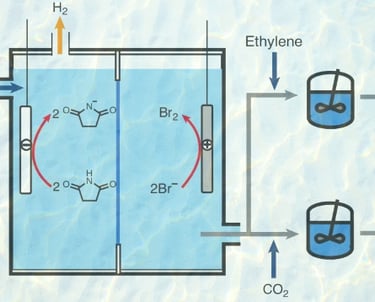
Nature Synthesis, 2024, 3, 846–857
A modular photoredox route towards sulfoximines
We report a modular photoredox strategy for the synthesis of aryl vinyl sulfoximines from sulfinamide and vinyl halide starting materials. This strategy demonstrates a difunctionalization of sulfinamides to sulfoximine products with excellent configurational retention and stereoselective trans alkenes. Under mild redox conditions, we propose the generation of a nitrogen centered radical that is resonance stabilized by a sulfur radical partner, increasing overall radical lifetime. This strategy we disclose is thus well suited for vinyl halide radical capture, leading to sulfoximine products. This process also entails broad modularity about the alkene, arene, and acyl protecting group, providing synthetic chemists multiple functional handles allowing for further exploration into the physicochemical properties that have shown promise in recent studies.
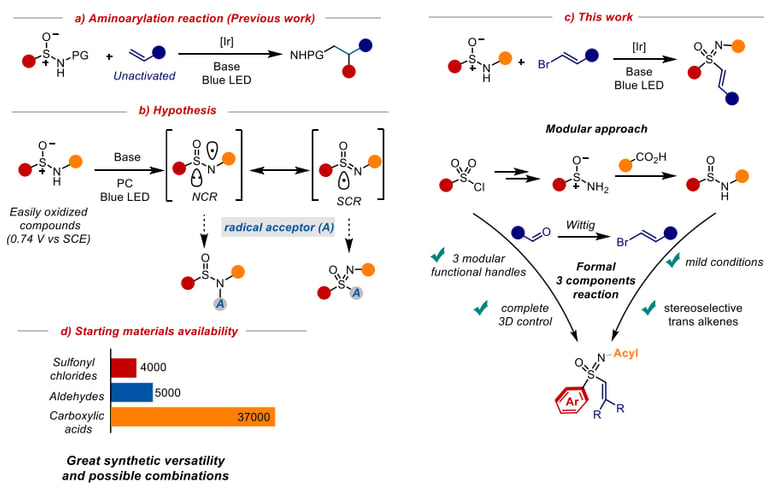
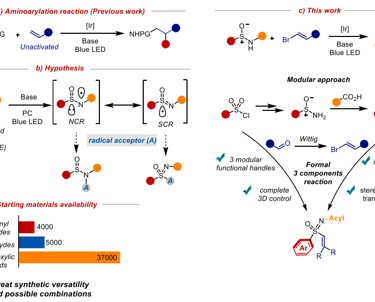
Chem Rxiv preprint
Read the paper
Synthesis of Bioactive Complex Small Molecule–Ciprofloxacin Conjugates and Evaluation of Their Antibacterial Activity
Here, we report a support-free CuI-nanoparticle-catalyzed strategy for conjugating electron-deficient and electron-rich terminal alkynes with a ciprofloxacin methyl ester. Our conjugation technique exploits the late-stage functionalization of bioactive natural products such as tocopherol, vasicinone, amino acids, and pharmaceuticals such as aspirin and paracetamol to provide conjugates in excellent yields under mild and green conditions. This protocol also enabled the synthesis of (hetero)arene-ciprofloxacin 1,4-disubstituted 1,2,3-triazoles in good yields and high regioselectivities. These synthesized ciprofloxacin conjugates were evaluated in vitro for their antibacterial activity against a panel of relevant bacteria. A significant number of conjugates showed comparable activity against Gram-positive and Gram-negative bacteria. Moreover, some conjugates exhibited less toxicity than ciprofloxacin against two mammalian cell lines, suggesting the utility for the future investigation of these compounds for in vivo efficacy and pharmacokinetic studies.


ACS Comb. Sci. 2020, 22, 440–445
Cu-Catalysed carboxylation of aryl boronic acids with CO2
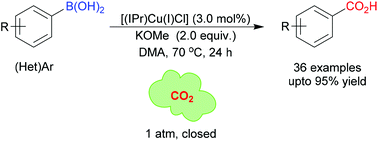

Org. Chem. Front., 2019,6, 3673-3677
Carboxylation of Alkenyl Boronic Acids and Alkenyl Boronic Acid Pinacol Esters with CO2 Catalyzed by Cuprous Halide
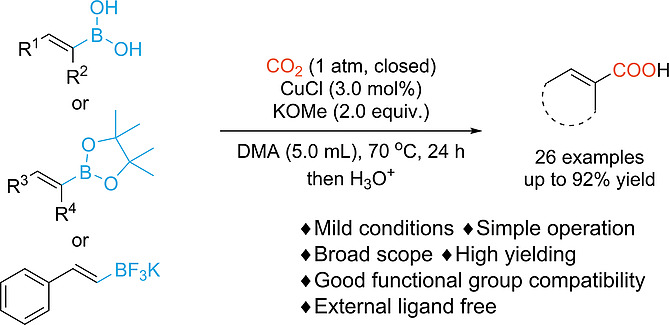
Eur. J. Org. Chem. 2020, 2020, 2813–2818
2-Aminoquinazolin-4(3H)-one as an Organocatalyst for the Synthesis of Tertiary Amines
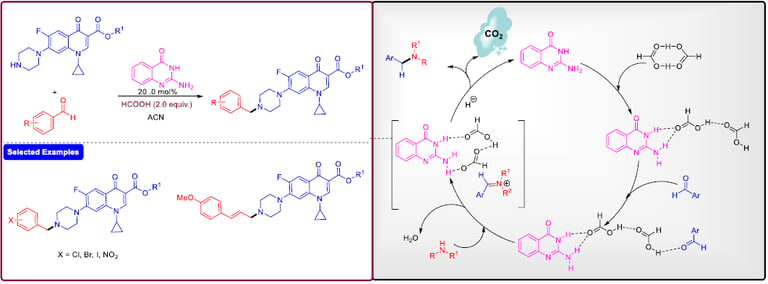
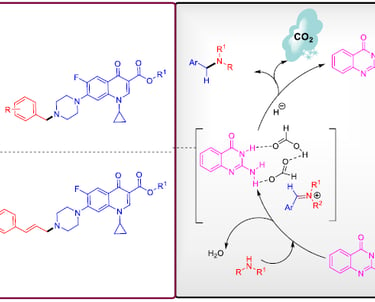
Org. Lett. 2018, 20, 5, 1359–1362
Tin-catalyzed selective reductive hydroamination of alkynes for the synthesis of tertiary amines.
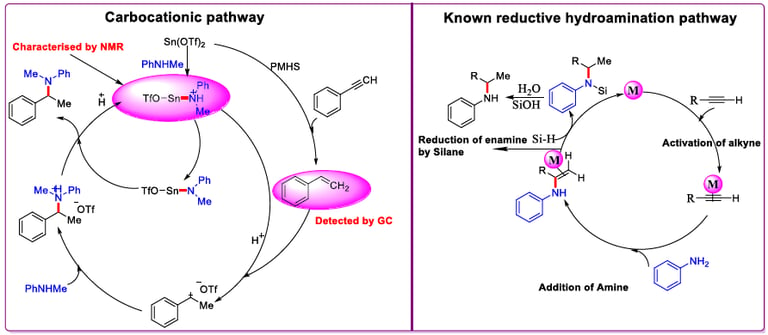
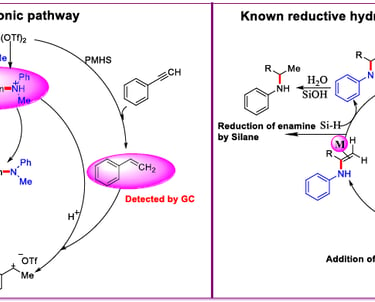
Adv. Synth. Catal. 2016, 358, 1103–1109
Synthesis of tertiary arylamines: Lewis acid-catalyzed direct reductive N-alkylation of secondary amines with ketones through an alternative pathway
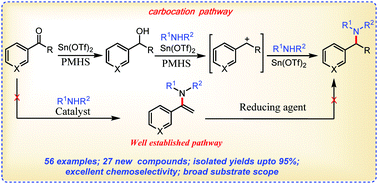

Chem. Commun., 2016,52, 9648-9651
CuI nanoparticles as recyclable heterogeneous catalysts for C–N bond formation reactions
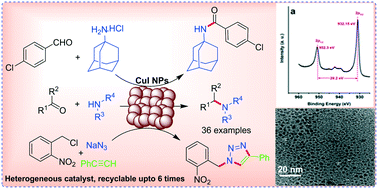

Catal. Sci. Technol., 2017,7, 2857-2864
Microwave assisted synthesis of phenanthridinones and dihydrophenanthridines by vasicine/KOtBu promoted intramolecular C–H arylation
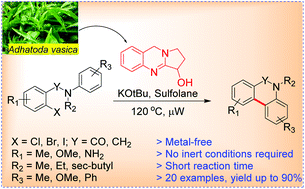

Org. Biomol. Chem., 2016,14, 8536-8544